In previous pages we've explored the chemistry and analysis of a variety of unstable radionuclides, typically in macro- or at least microscopic- quantities. This page is devoted to the analysis of traces of these radionuclides in nature. Some of them, like Iodine-131, are short-lived markers indicating the release of manmade nuclides into the environment; others like Cesium-137 are anthropogenic, but longer lived tracers of human's effect on the environment. Finally some isotopes, like Beryllium-7 occur without any intervention at all, the result of active atmospheric synthesis at the edge of space. Like we explored with nuclear forensics, these radioactive atoms can be powerful tools to understanding important questions, from the proliferation of nuclear weapons, to the effects of nuclear accidents on the environment, even as tracers to follow changes in the soil, air, and water of our planet.
The accident at the Fukushima Daiichi Nuclear Power Plant
I have been interested in following the radioactive fingerprints of nuclear fission in the environment from the earliest days of my involvement with nuclear science. Being born in the mid-nineties, I missed many of the important releases of anthropogenic radionuclides into the environment, the Chernobyl accident in April of 1986, and US and Russian atmospheric nuclear testing from 1946 to 1963 when concerns over the environmental impacts of round-the-clock nuclear tests lead to the ratification of the Limited Test Ban Treaty (LTBT) banning all nuclear tests in the atmosphere, oceans, and outer space.
On March 11, 2011, Japan, and the world at large, watched in fear at a devastating series of events following a massive M9.0 earthquake off the eastern coast of that country. A nation with a modern and extensive nuclear power program was crippled first by the movement of the ground and then a rapid influx of water from the subsequent tsunami. The Fukushima Daiichi Nuclear Power Plant on the northeast coast of Japan sustained significant damage after the disaster, with 3 of its 6 Boiling Water Reactors (BWRs) at power when the earthquake struck. Although a shutdown, or SCRAM, of the reactors was initiated because of the earthquake, subsequent inundation from the resulting tsunami led to the loss of offsite and on-site emergency backup power generation required to remove decay heat and keep the fuel assemblies in the reactors cool. Over the next several days, melting of the reactors' fuel combined with large explosions from the combustion of hydrogen gas released a large fraction of the volatile radionuclide inventory of the reactors into the environment, leading to the worst nuclear power accident since the Chernobyl disaster in 1986.
8.02 day Iodine-131 and 3.2 day Tellurium-132 in a sample of snow collected in Reno, NV after the accident.
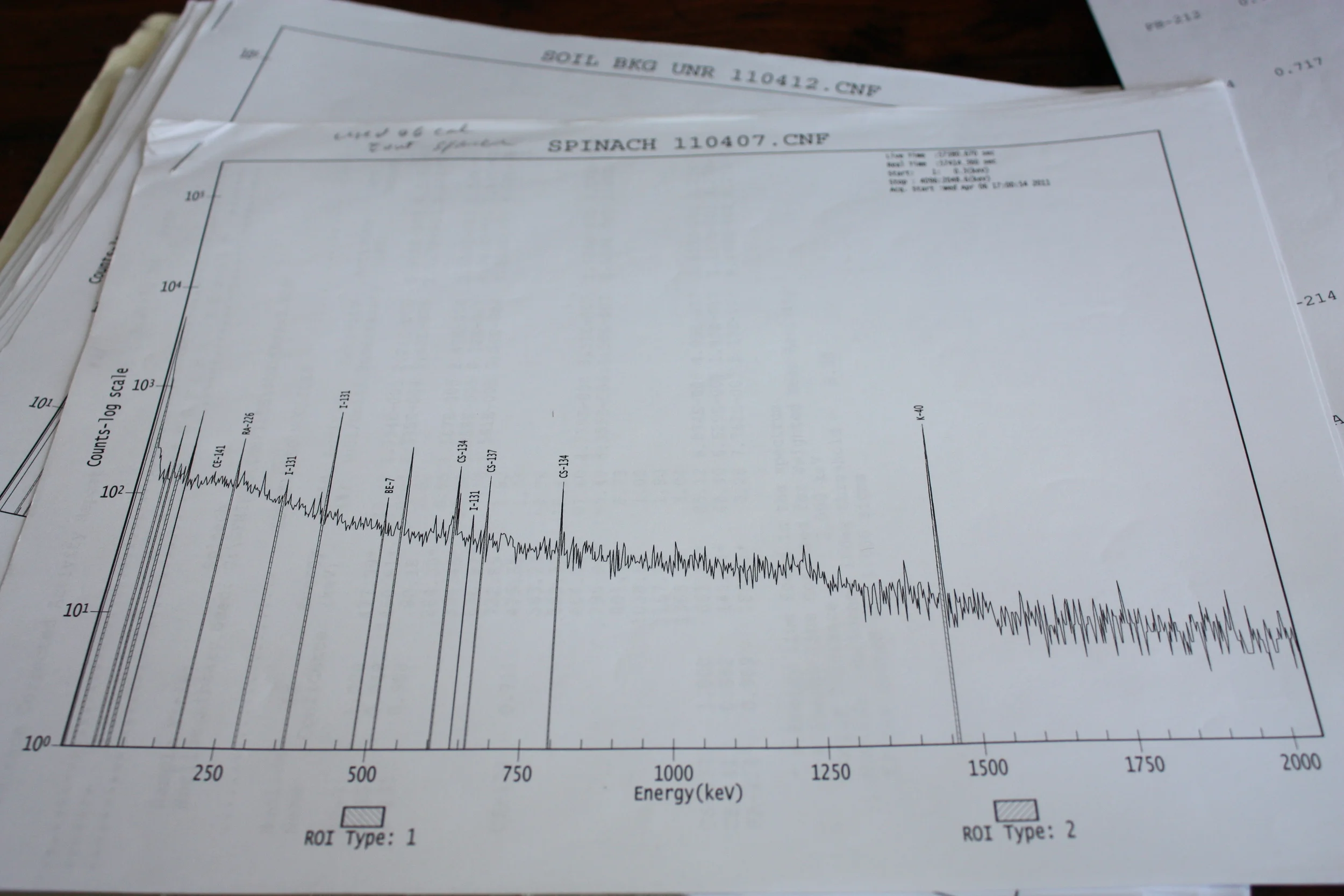
A sample of spinach grown in California showing traces of the Fukushima accident, including Cesium-135, Cesium-137, and Iodine-131, along with naturally occurring Potassium-40 and Beryllium-7.
In the days following the accident, I quickly decided to setup equipment to monitor for the inevitable traces of the accident that would soon arrive in the atmosphere over the western United States. I was curious to qualify the presence, and the quantify the amount of these radionuclides not only to view their impact on and distribution in the local environment, but to provide clues as to the nature of the condition inside the reactors in the absence of more thorough reporting from the site. In the weeks that followed, I was able to quantify the levels of many radionuclides from my lab in Reno, NV, including isotopes of Cesium, Iodine, and Tellurium. By analyzing the nuclides detected, and their quantities, information on the scale of the accident could be gleaned from comparisons with previous large scale releases of materials. The presence of large quantities of Iodine-131 suggested that significant fuel damage had occurred and that, whether through operator input or structural deficiency, the containment barriers for this volatile radionuclide had been compromised. Additionally the presence of radionuclides of Cesium provided a more complete picture of the severe damage to fuel assemblies and the possibility for longterm radio-ecological impacts to the local environment in Japan. However, the lack of many less volatile nuclides seen the plume from the Chernobyl accident also gave hope that there was some containment of the source term of the three damaged reactors and spent fuel at the Fukushima Daiichi site.
Today my interest lies in tracking the radionuclides released during the accident and from accidental discharges today in the Japanese environment. The identity, size, chemical form, and environmental behavior are all important factors to successfully mitigate the impact of the reactor accidents. Today, the main radionuclide of concern in the prefectures surrounding the damaged power installation is Cesium-137, with a half-life of 30.1 years. The important piece of good news in Japan today, several years after the accident, is that radionuclides exhibiting much higher radiotoxicity, including the bone-seeking beta emitter Strontium-90, and long-lived actinide alpha emitters like Plutonium, were not released in large quantities in the environment outside the site. While I continue to look for and monitor the presence of these nuclides as time passes, it appears that Cesium-137 is the nuclide of priority in determining clean-up and rehabilitation of areas affected by the accident. Effective measures for cleaning soil, reducing external dose-rates, and preventing the incorporation of this radionuclide into agricultural and food products should be of primary focus going forward.
Fingerprints of Chernobyl
On April 26, 1986, a combination of reactor design and operator error lead to the world first significant nuclear power disaster in the Soviet Union. Lacking a formal containment structure and with significantly quantities of fuel exiting the core of Unit 4, the accident released large plumes of radionuclides across Europe and across the globe. The large metropolitan area of Kiev, Ukraine was spared heavy fallout due to wind direction, but wind and most importantly precipitation washed out this plume in various locations throughout Europe, with Belarus being the most heavily impacted. While many of the nuclides most important to understanding the public health impacts of the disaster like Iodine-131 have a short residency in the environment, a few including Cesium-137, Strontium-90, and Plutonium-239/240 persist from decades and centuries later. Cesium-137 from the Chernobyl plume remains problematic in many parts of Europe, with the contamination of farmland and foodstuffs a concern to authorities tasked with limiting dose to the public from consumption of these items. Germany for example, has problematic radioactive wild boars, who feast off truffles which tend to have a high affinity for Cesium. So do many types of berries, an example of a Forest Berry jam, produced in Austria, and imported into the US, is shown at right. The Cesium-137 content of this Jam, while not harmful or in excess of interventional limits, exists today as a fingerprint of Chernobyl from nearly 30 years in the past.
Cesium-137 in the soil
Beryllium-7 and cosmogenic radionuclides